“The meeting agreed to give conditional registration approval for emergency use to the ‘Spikevax 0.20 mg/ml Dispersion for Injection’ mRNA vaccine (nucleoside modified) product,” said the DG. The Moderna vaccines acquired by Malaysia will be manufactured by Rovi Pharma Industrial Services in Spain. It’s not yet known whether the US-developed jab will be included in the National COVID-19 Immunisation Programme (PICK) or go straight to the private market, which had recently received authorisation from the government to sell the vaccines.
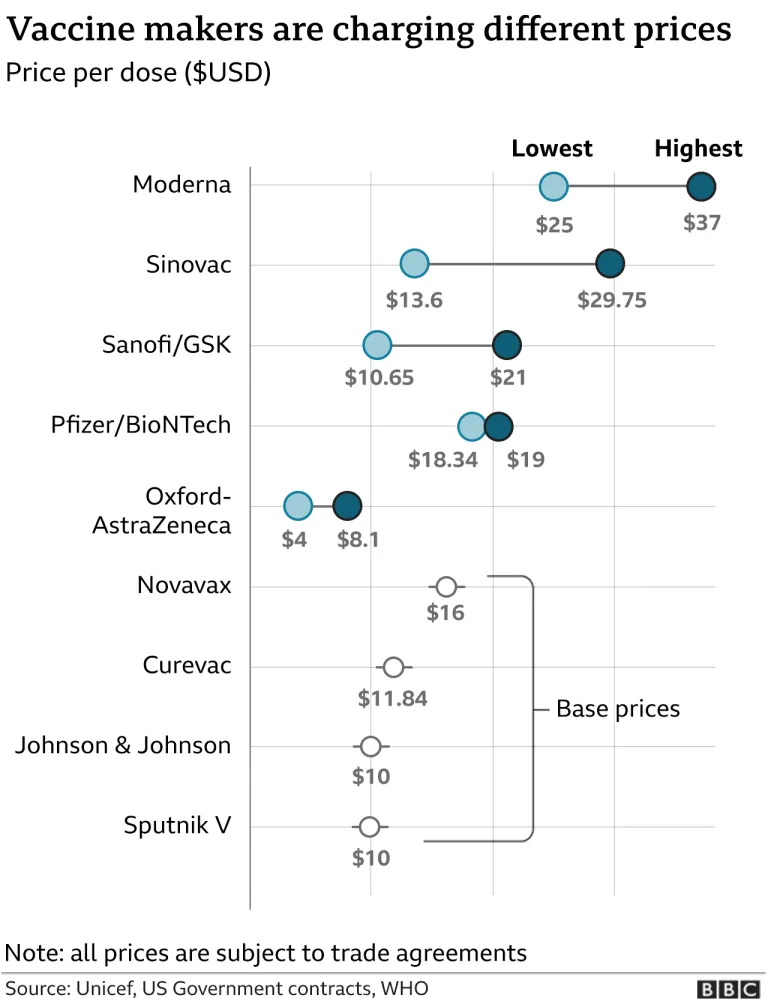
The Moderna COVID-19 vaccine, also known as mRNA-1273 or Spikevax, is the second mRNA vaccine to hit the market after Pfizer’s. It’s stored between -50°C and -15°C, just slightly above Pfizer’s ultracool requirements — although Moderna is working on a new version of the vaccine that can be safely kept using a refrigerator instead of a freezer. Spikevax boasts an efficacy rate of 94% and on Thursday, it revealed data that showed recipients retained protection of 93% against the virus even after six months. The data compares favourably to Pfizer’s mRNA shot which decreased to 86% effectiveness after the same time period. Khairy Jamaluddin, PICK coordinating minister, revealed back in January that the government would not be adding the Moderna vaccine to its portfolio due to it being too expensive, saying that procuring the vaccine would put the programme over its budget. Earlier this week, both Moderna and Pfizer implemented a price hike for their respective COVID-19 vaccines; where the former increased from EUR 19 (~RM 94.80) to EUR 21.50 (~RM 107) per dose, while the latter rose from EUR 15.50 (~RM 77) to EUR 19.50 (~RM 97). (Sources: KPK, Reuters [1][2], CDC, Codeblue, Forbes // Images: Mike Segar/Reuters, BBC)